The answer key for CBSE Class 10 science theory board examination held on March 04, 2020. The solved question paper is prepared by subject experts
Question paper series- Set1- 31/4/2
Download the question paper from CBSE Class 10 Exam 2020 Question papers
[button type=”default” color=”green” target=”_blank” link=”https://www.educationobserver.com/cbse-10th-social-science-exam-2020-question-paper-and-solutions/”]CBSE Social Science Question Paper and Solutions [/button]
[button type=”3d” color=”green” target=”_blank” link=”https://www.educationobserver.com/cbse-10th-science-exam-2020-question-paper-and-solutions-set-2/”]New !! CBSE 10th Science Solved Paper 2020- SET 2[/button]
[button type=”3d” color=”green” target=”_blank” link=”https://www.educationobserver.com/cbse-10th-maths-exam-2020-question-paper-solutions/”]New !! CBSE 10th Maths Solved Paper 2020[/button]
Science Paper Solutions
Ans 1. Vegetable oils containing unsaturated fatty acid are good for health
Ans 2. The current produced by the phenomenon of electromagnetic induction when a coil is placed in region where magnetic field changes with time.
Ans 3 a. This law states that when elements are arranged in the order of increasing atomic masses the elements with similar properties occur at regular interval. The properties of elements are periodic function of their atomic masses.
Ans 3b. Mendeleev left some gaps for the undiscovered elements like gallium, scandium and germanium when these elements were discovered later on, they were placed in those gaps without disturbing the exciting elements.
Ans 3c. RH4, R02
Ans 3d. (i)
Ans 4a. The two methods of effective plastic waste collection in school are:
- Curb side Recycling
- Drop off Recycling
Ans 4b.
1. Plastic Grocery Bags
2. Plastic Beverage Bottles
Ans 4c. Environment friendly substitution can be provided in the following ways
1. By making paper bags
2. By providing cloth and Jute bags.
Ans 4d) No, in landfills. The microbes will break the waste naturally and in labs, they will work in controlled manner.
Ans 5. (a)
Ans 6. B or B
Ans 7. C
Ans 8. B or B
Ans 9. A (5*1/5=1 0hm) Or B
Ans 10. C
Ans 11. B
Ans 12. D
Ans 13. C
Ans 14. A
Ans 15a. The type of reaction mentioned above is double displacement reaction.
Ans 15b. The colour of solution fade away due to formation of copper sulphate.
Ans 15c. The chemical name of the black precipitate formed is copper.
Ans 15d. CuS04 + H2S –> CuS + H2So4
Or
- The process is electrolysis.
- Anode and Cathode are made of Carbon.
- 2H2O –> 2H2 + O2
- H2SO4 will make water a good conductor of electricity.
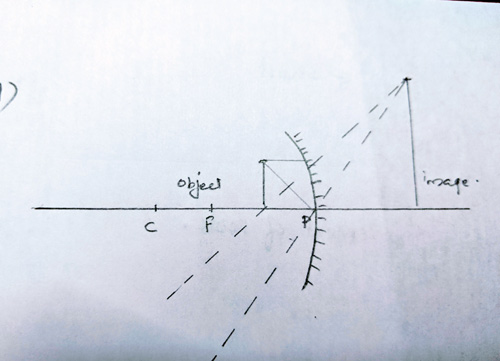
Ans 16 a. The image will be formed behind the mirror.
Ans 16 b. Size of the image will be larger than the object.
Ans 16 c. Image is erect and virtual
Ans 16 d. Labelled Ray Diagram:
Ans 17. The gradual unfolding of organisms from pre-existing organisms through changes since the life began. We cannot equate evolution with progress because evolution depends upon the accumulation of variation. These variations must be useful also.
Or
Similarities among the forelimbs of human, birds and bat point out their common ancestry. Forelimbs perform different functions in all above mentioned cases.
Ans 18.
| Pituitary gland | Located just below brain | Controls growth of body |
| Thyroid gland | Attached to windpipe | Controls the rate of metabolism of carbohydrates fats and proteins. |
| Pancreas | Just below stomach | Controls the blood sugar level IT controls metabolism of sugar. |
Ans 19.
| Galvanization | Alloying |
| 1. This process of depositing a thin layer of zinc ok on iron object. | 1. Alloy is homogeneous mixture of two or more metals and small amount of iron metal. |
| 2. It is done by dipping an iron object in molten zinc. | 2. It is prepared by mixing the various metals in molten state. |
| 3. Thin layer of zinc is deposited on iron object which prevents it from rusting. | 3. The proportion of metals and non-metals can be changed. |
Or
Sodium react with cold water and tremendous amount of heat is released. H2 gas evolved catches fire.
2Na + 2H2O –> 2NaOH + H2 + Heat
Calcium too reacts with cold water. But here, H2 gas evolved does not catch fire.
Ca + 2H2O –> Ca(OH)2 + H2
Magnesium metal does not react with cold water. It reacts with hot water to form magnesium hydroxide and hydrogen.
Mg + 2H2O –> Mg(OH) 2 + H2
Ans 20. Carbon is able to form so many compounds because of the following reasons:
- It is small in size.
- Self-combining property known as catenation property.
- Tetra covalence.
As the size of carbon is small, so H can combine with other elements and form so many compounds full stop also the force between the compound is too strong, so it cannot be broken easily.
Ans 21. Geotropism is the movement of plant parts in response to the gravitational pull of the earth.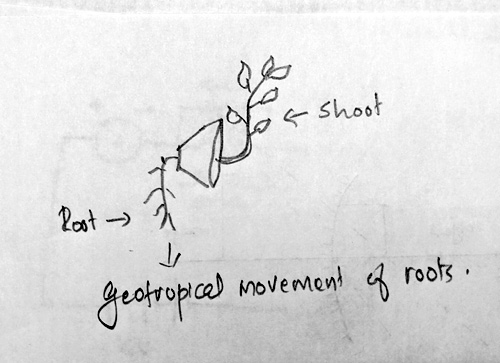
Ans 22a. Out of the two, P is optically denser as the angle of refraction is less (20o). So it will bend more towards the normal ray.
b) One dioptre is the power of a lens whose focal length is 1 metre. (P= 1/F)
c) P = 0.5 D 1/F=0.5 D or 1/0.5 =F (2m=F)
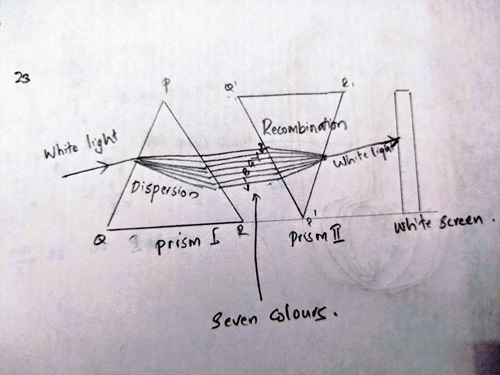
Ans 23. The two prisms will be recombined to get a narrow beam of white light. First prism through which light passes first will be placed on its base and another similar prism is placed alongside it in inverted position on its vertex so that its refracting surface is in the opposite direction.
Ans 24a. This condition is called Presbyopia.
Ans 24b. This condition happens due to weakening of ciliary muscles. Eye lenses become inflexible. The eye loses its power of accommodation.
Ans 24c. A person suffering from this defect will have to wear spectacles having bifocal lens in which upper part is concave lens used for distant vision and lower part is convex lens used for reading purpose.
Ans 25. An alloy is a homogeneous mixture of two or more metals or metal and small amount of non-metal. Alloy is prepared by mixing the various metals in molten required proportion and then cooling the mixture to room temperature. Alloy of metal and non-metal is prepared by first melting the metal and dissolving the non-metal in it followed by cooling to room temperature.
Its advantages are:
1. Alloys are stronger than the metal from which they are made.
2. Alloys are more resistant to corrosion.
Composition of Stainless Steel Carbon(0.1% to 1.5%) mixed with iron
Reason for using steel are:
1) It is hard and strong
2) It rusts less readily than pure iron
Ans 26. Diagram:
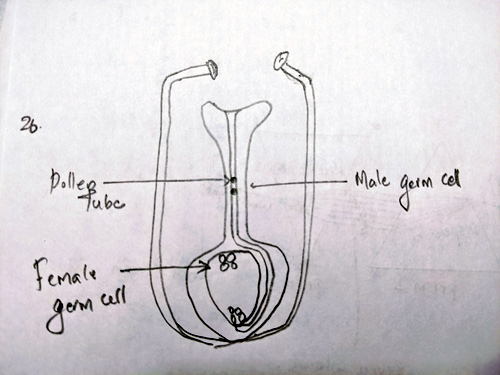
Pollen grains are transferred from one another to the stigma of flower. This is known as Pollination
As the pollen grains are transferred on the stigma they release to male gametes which form a pollen tube to the ovary by the process of chemotropism.
As the reach the embryo one of the male gamete, fuses with one female gamete to form zygote. This is called fertilization.
The other male gamete fuses with two of the female gametes and form of polar nuclei. This is called double fertilization.
The zygote develops into embryo and the polar nuclei develops into embryo sac.
They both together form the bud.
OR
- The age at which the sex hormones begin to be produced and the boy and girl become sexually mature (able to reproduce) is called Puberty.
- i) Testes are primary reproductive organs in male. The function of testis is to prepare male gametes called sperm.
- ii) Seminal vesicle: These secrete liquid into the sperm which is moving Vas defens. It acts as nutrition to sperm and help in its motility.
iii) Vas defens: It carries sperm produced by testis and joins to Urethera.
- iv) Urethera: It is a common passage for sperm and Urine. It carries the sperm to Penis that passes the sperm from man’s body into woman’s body.
- The testis is outside the abdominal cavity because sperm formation requires a lower temperature than normal body temperature. The temperature of scrotum is 3Oc less than body temperature.
- The sperm is released into vagina at one time. The sperm moves through the cervix into the Uterus.
Ans 27 .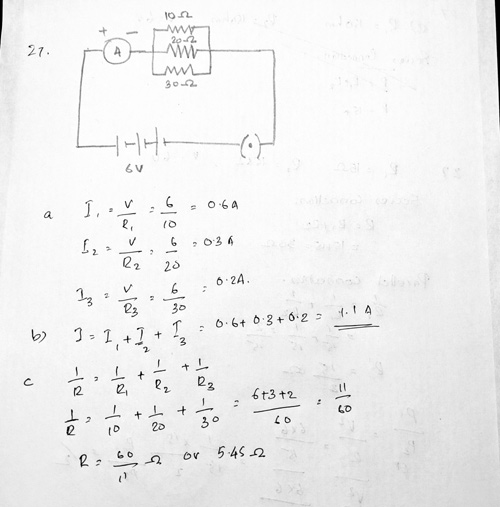
Ans 27 OR
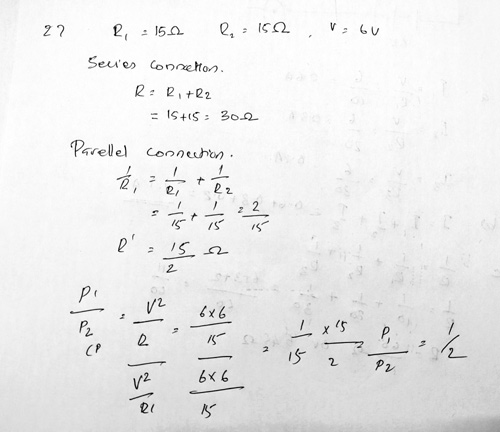
Ans 28. The indicator used is olfactory indicates as the odour is changed. Since liquid K is an acid.
- When ‘X’ reacts with Zinc granules then salt and H2 gas is formed.
X+ Zn –> Salt of Zinc + H2
- X + Na2Co3 –> Salt of Sodium + CO2 +H2O
Salt of Sodium, Carbon Dioxide and water is formed.
Ans 28. OR
The water molecule that forms part of the structure of crystal are called water of crystallization. These are also known as hydrated salts. Every hydrated salt has fixed number of molecules of water of crystallization.
E.g. NaCo3. IoH2O (Washing Soda Crystal)
CaSo4.2H2o (Calcium Sulphate Dihydrate)
Water of crystallization give the crystals of the salt their shape and in some cases imparts them colour.
When hydrated salts are heated strongly they lose their water of crystallization. They lose their regular shape and colour.
CuSo4.5H2O –> CuSo4. + 5H2O
When water is added to anhydrous copper sulphate it gets hydrated and turns blue.
CuSo4. + 5H2O –> CuSo4.5H2O
Ans 29a. Nutrition is necessary for human body for energy e to perform various activities for growth and repair of cells and tissues.
Ans 29b. The rhythmic contraction and relaxation of food pipe known as peristaltic movement is responsible for movement of food inside the alimentary canal.
Ans 29c. Small intestine is herbivorous and is larger as compared to carnivorous as longer intestine allow the cellulose present in grass digest completely and meat is a food that is easier to digest
Ans 29d. The function of mucus is to prevent the stomach wall full stop if mucus is not secreted in the stomach walls will get damaged.
Ans 30a. All electrical appliances in common domestic electric circuits are connected in parallel because of the following reasons:
1) All appliances will be operated by different switches.
2) If one appliance stop working it will not affect another.
3) All appliances will get same voltage.
Ans 30b. The two different circuits in a house lighting circuit with 5 A fuse and power circuit with 15 A fuse. Lighting circuit is for running low power rating devices such as electric bulbs, fans, radio etc. Power circuit is for running high power rating devices like Electric iron.
Ans 30c. Electric short circuit – if insulation of the live wire and neutral wire gets stone then the two wires touch each other. Live wire and neutral wire come in contact with each other. current flowing through the wires become very large and hits the wire to a high temperature and fire may start.



NIKKKUUUU
CHALAK SAA HAIII
4d) answer is yes… Microbes will work similarly in landfill sites as they work in laboratories as its their nature to decompose and they will work similarly wherever they are.
I love you
Yes, you are right answer is 1:4
Thanks for solutions
And:-5 is wrong it is option B
Thanks no becoz I search set 2 then answer of set 1
I think answer for que 27{option} must be 1:4 not 1:2…..
Yes, you are right answer is 1:4
Please release science set3 code 2/4/3 solved paper
Please post full solved paper for maths set2
Please also post set3 ?????
Ťhåñķ§ ä ľøť
Thanks education observer for sharing this. Its really helpful for students, Please also share some tips and suggestions for top schools in Gurgaon for improving the education system.
Thank you … Education observer is the best??????
It’s 1:2
Question paper series- Set1- 31/5/1 solution
Yes 27 question is 1:4
For the 27th ques, the answer is 1:4
Set -2 code – 3/3/2 answers upload kr liye
Please give the solution of set 1 code no.31/3/1.
Please upload other set questions answer keys
Thank you….. At last one reliable answer key released. Thank you educationobserver.com